Over the past two days we have discussed diverse topics related to global health security at the 2024 Emerging Leaders in Biosecurity (ELBI) Symposium in Washington DC. One round table discussion we had focused on the question of how countries should share data before, during, and after a public health emergency?
I previously examined this topic while studying national COVID-19 data reporting systems from African countries. Our study revealed multiple insights into the diversity of ways that data are shared during a public health emergency: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9237488/
During the COVID-19 pandemic, gaps in data made it challenging to develop timely predictions and counter-measures. There were many formal and informal channels for sharing national data, and data varied in terms of frequency, format, and content. This led to requests by scientists and policymakers for greater standardization and accountability for international data reporting.
One way of framing the ongoing discussion on data sharing is to understand what types of data need to be shared.
A main focus of the discussion on data sharing has centered around biological samples and the WHO Pathogen Access and Benefit-Sharing System as part of the Pandemic Accord negotiations, and this is an important part of addressing global health equity. A less discussed area, but also important for global health equity and security, is the sharing of clinical and epidemiological data.
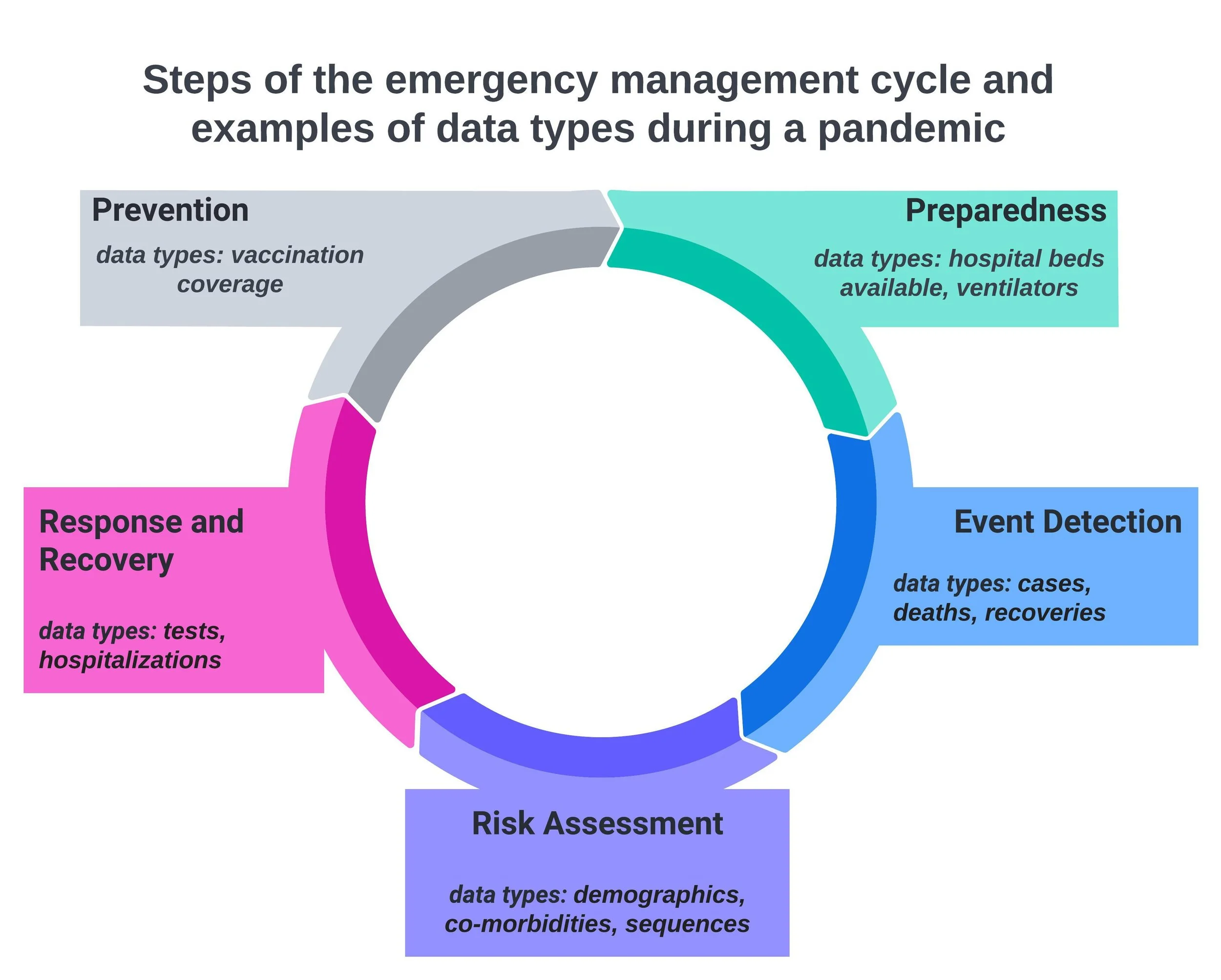
The emergency management cycle, which I have illustrated below, can be a helpful way of thinking about the different types of clinical and epidemiological data during a pandemic or other public health emergency. The steps of the cycle include: prevention, preparedness, event detection, risk assessment, response, and recovery. Each step of the cycle is associated with different types of data.
In our research we found that many countries shared event detection data, such as cases and deaths, but fewer shared risk assessment or response data such as co-morbidities and hospitalizations.
So how do we improve data reporting during future pandemics and public health emergencies?
The International Health Regulations (IHR), which include data reporting requirements for WHO member states, were recently revised and agreed upon on June 1st 2024 (recent revisions to the IHR are in bold in the document):
https://apps.who.int/gb/ebwha/pdf_files/WHA77/A77_ACONF14-en.pdf
The revised IHR states that “essential information” to report during a health emergency includes: “clinical descriptions, laboratory results, sources and type of risk, numbers of human cases and deaths, conditions affecting the spread of the disease and the health measures employed.”
In this section the IHR appears to be more specific about the kinds of event detection and risk assessment data that countries must share. There is need for further clarification and discussion on how countries should report prevention, preparedness, and response/recovery data. For example, during the COVID-19 pandemic, hospital bed availability was a key metric that researchers used to predict healthcare capacity and help guide decision-making for policymakers.
Therefore, as the Pandemic Accord negotiations continue and we reflect on the lessons from COVID-19, there is a need for further emphasis on what types of data must be shared, as well as how this information should be shared to ensure a swift and equitable global response.